Tel: (office): (925) 480-7497
Tel: (mobile): (925) 413-7297
Email: aretzios@adrclinresearch.com
Target Product Profiles
An Essential Tool for Planning Clinical Studies and Evaluating
Candidates for Further Development
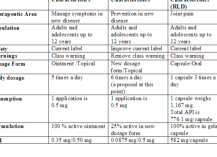
Target Product Profiles are an essential tool in the development of novel drugs, biologics and devices. They allow for strategic planning and management and help focus the attention of the organization on the key aspects of each project. TPPs assess the opportunity, determine the risks, and prompt the design of mitigation plans. A short presentation on the assembly and utilization of Target Product Profiles can be found here. Anastassios D. Retzios has authored the chapter on “Target Product Profiles” in RAPS’ “Global Pharmaceutical and Biologics Regulatory Strategy, Second Edition ( May 2020)”. This textbook provides up-to-date information on the process of development and approval of drugs and biologics.Download Presentation (PDF)


Copyright© 2021 - Bay Clinical R&D Services - All Rights Reserved




















