Tel: (office): (925) 480-7497
Tel: (mobile): (925) 413-7297
Email: aretzios@adrclinresearch.com



Services
Comprehensive Regulatory and Clinical Development Strategy
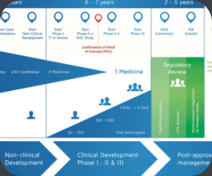
We can examine the regulatory precedent and the competitive environment to definte the best approach to obtain marketing authorization at the earliest time with the proper indication. In this process, we develop a detailed target product profile that takes into account the current competition, if any, as well likely competitors in the proximal future. We will identify the optimal regulatory route and key milestones. We will develop protocols for all phases of clinical development (and the supporting documentation such as investigators brochures) which will clearly support all the goals of your project.Target Product Profile (TPP) Development
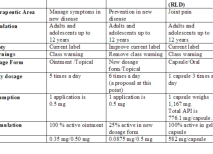
We help design the drug/biologic/device development program on the basis of the label claims that you are targeting. The utilization of Target Product Profiles (TPPs) assists in outlining the clinical program required, provide detailed information on risk and remediation strategies and provide choices regarding the likely marketing opportunities. A short presentation on the utility of TTPs is attached (Download Presentation)Product Portfolio Review
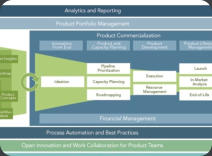
We can help you understand the strength and weakness of your portfolio at any stage of your development, the potential risks as well as the opportunities and the possible avenues of development to help you concentrate resources in the appropriate area. This effort incorporates Target Product Profiles, existing product labels, marketing research for the potential competitive landscape and other development strategy toolsClinical Research and Development
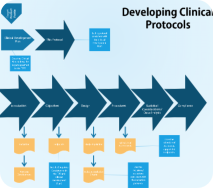
We have extensive experience in designing clinical studies and authoring the appropriate clnical protocols and satellite documentation. can assist in writing or amending clinical protocols, inverstigators brochures, study safety and data collection plans and other documents essential in the clinical development of your drug or biologic. If required, we can support our choices for sample size, study population, inclusion and exclusion requirements, primary and secondary endpoints and types of analyzes to regulatory agencies in appropriate communications. We can author clinical study reports or work with your medical writing group to reinforce their key points and conclusions.Clinical Operations Management
The successful management of clinical operations management is crucial for any program. We can help you with the conduct of operations at any stage of clinical development. We can assist in the selection of CROs and other vendors, conclude agreements that would support the timeline and budget of your organization, coordinate CROs in a variety of countries (US, Far East, India, West and East Europe, Latin America), interface with clinical investigative sites, put specific guidelines in place to achieve reliable and accurate data collection and manage the overall program within the constraints of your timeline and budget.Due Diligence
We have built extensive experience in due diligence and have assisted in the acquistion or in placing bids on specific drugs and biologics by a number of companies. Therefore, we can be of substantial assistance in determining and documenting any potential issues that may lurk in the development process of the products that you may be interested in acquiring or in-licensing. Our approach to due diligence is outlined in “Due Dilgence Considerations” (Download Document)Organizational Matters (Clinical Department Organization,
Training and Quality Assurance)
We can help evaluate the processes and procedures of the clinical department, analyze the gaps and develop and author standard procedures (SOPs), organize training to bring the department into full compliance
Copyright© 2021 - Bay Clinical R&D Services - All Rights Reserved




















